

KINGSPORT, Tenn., Jan. 30, 2018 /PRNewswire/ -- Global specialty plastics provider Eastman demonstrates the dominant performance of next-generation material Eastman MXF221 as a disinfectant-ready polymer for safer healthcare environments at the Medical Design & Manufacturing (MD&M) West trade show in Anaheim, California, Feb. 6 to 8. The latest advancement in biocompatible medical-grade polymers for medical device housings and hardware, Eastman MXF221 copolyester offers unsurpassed chemical compatibility with stringent disinfectants used to combat healthcare-associated infections (HAIs) as well as improved durability and impact strength.
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8261151-eastman-disinfectant-ready-polymer-mdm-west/
"Today's healthcare environment has a critical need for better plastics—and better plastics testing, selection and design," said Ellen Turner, Eastman global market development manager, medical devices. "As the market and regulatory landscape is ever-evolving, device manufacturers have to consider material capabilities at every stage of product development. Eastman surrounds brand owners with service throughout their product development process, providing invaluable assistance to brand owners as they develop equipment that meets requirements for clinical settings."
Eastman plastics are used to make key components in non-implantable medical devices, including syringes, minimally invasive surgical instruments, blood contact systems, rigid and flexible tubing, IV components, and renal devices. Eastman MXF221 is a fully compounded polymer uniquely suited for electronic medical device housings and hardware due to its inherent ability to withstand stickiness, discoloration, crazing, cracking and hazing.
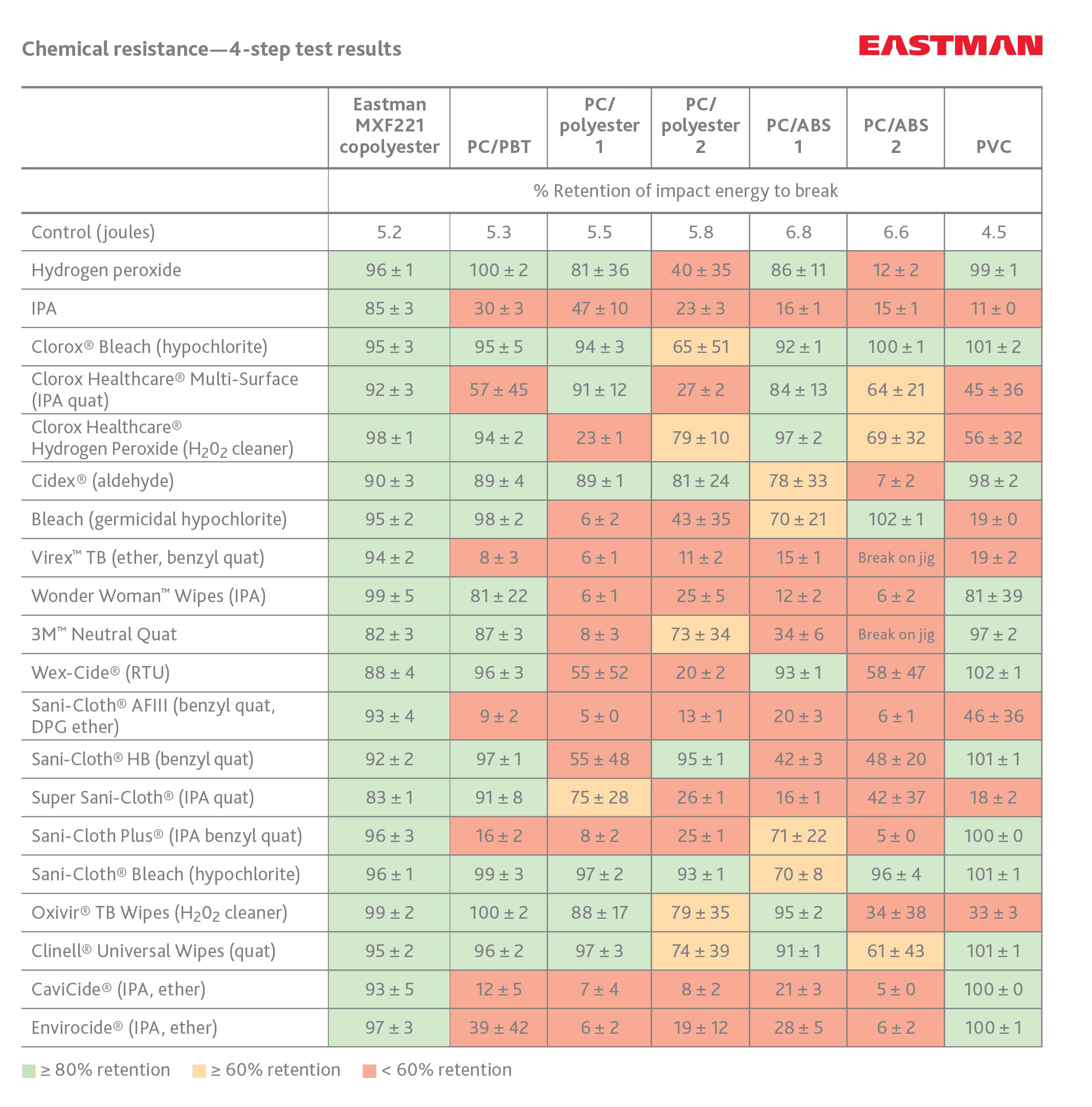
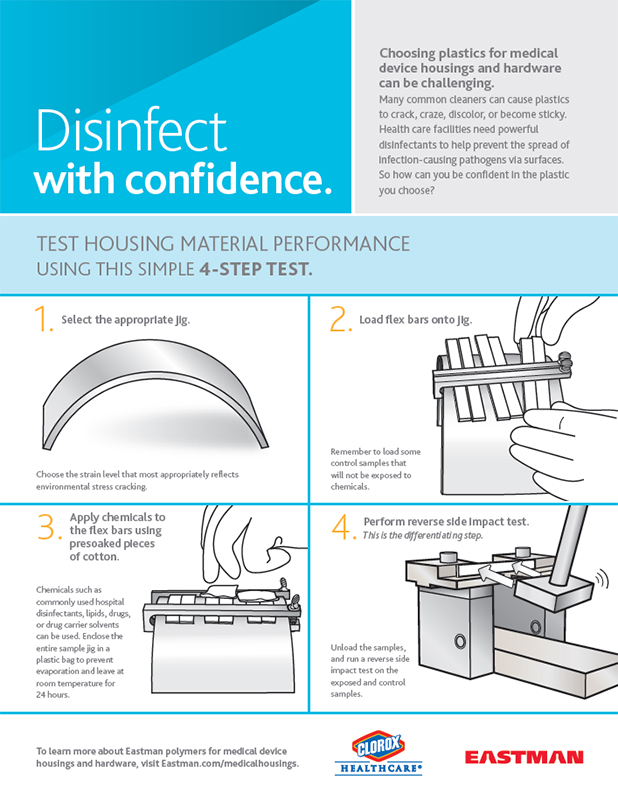
Eastman MXF221 performs better than other materials used in medical devices and equipment as evidenced in the findings of Dr. Yubiao Liu, applications development associate for Eastman, who developed the simple, replicable four-step test to evaluate long-term resistance of plastics to hospital disinfectants. Eastman MXF221 offers unsurpassed chemical resistance to today's disinfectants used in new cleaning protocols needed to prevent HAIs. Eastman MXF221 retains more than 90 percent of its original impact strength after exposure to stringent disinfectants—far superior to competing materials such as polycarbonate blends.
Proactively selecting the right polymer for medical devices and housings can prolong the life of devices, improve reliability and save hospitals money in the long run," Turner said. "As a multi-material solution provider, Eastman is committed to helping our customers choose the right plastic to meet their product requirements."
In addition to Eastman MXF221 for device housings, Eastman's family of brands includes Tritan™ copolyesters for devices, Eastar copolyester 6763 and Eastalite copolyesters for rigid medical packaging, Ecdel elastomers for pharmaceutical packaging and Eastman 168™ non-phthalate plasticizer.
About Eastman Chemical Company
Eastman is a global advanced materials and specialty additives company that produces a broad range of products found in items people use every day. With a portfolio of specialty businesses, Eastman works with customers to deliver innovative products and solutions while maintaining a commitment to safety and sustainability. Its market-driven approaches take advantage of world-class technology platforms and leading positions in attractive end markets such as transportation, building and construction and consumables. Eastman focuses on creating consistent, superior value for all stakeholders. As a globally diverse company, Eastman serves customers in more than 100 countries and had 2016 revenues of approximately $9.0 billion. The company is headquartered in Kingsport, Tennessee, USA, and employs approximately 14,000 people around the world. For more information, visit www.eastman.com.

Editorial Contact:
Laura Mansfield, APR
The Tombras Group
+1 (865) 599.9968
lmansfield@tombras.com
SOURCE Eastman