


In the news release, HeartStitch® "Sutures The Future" at the TCT in Denver, Colorado, issued 20-Nov-2017 by Nobles Medical Technology II, Inc. over PR Newswire, we are advised by the company that the HeartStitch® Inc. boilerplate and logo should be included with the release. The complete, corrected release follows:
FOUNTAIN VALLEY, Calif., Nov. 20, 2017 /PRNewswire/ -- TCT 2017 in Denver, Colorado, provided a forum for Interventional Cardiologists from around the world to discover the HeartStitch® Suture-Based Solutions to Structural Heart Procedures. These key procedures of interest include septal repair, mitral and tricuspid valve repair and new access and closure techniques, all areas HeartStitch® has developed suture-based technologies. With significant demand for the NobleStitch™ EL suture closure system throughout Europe and Central Asia for PFO, ASD closure and ASA repair, many of its key customers have expressed their interest to expand the use of the HeartStitch® technologies to the valves and other structural heart defects. In the US many Physicians acknowledged being aware of the suture-based closure system, however a considerable shift was noticed at this year's conference in light of many recent positive publications on PFO closure including the presentation by Dr. Achille Gaspardone on the Italian NobleStitch™ EL data, which demonstrated equivalent or better closure rates than the competitive umbrella data and better safety data. Interest in availability of the product and desire to have training hit a new peak.
Dirk Segers, VP of European Sales and Marketing noted, "The traffic we found this year took a noticeable turn. In the past years at TCT, we had quite a bit of 'investigative' interest - physicians wanting to see what the technology was, asking about ease of use and certainly examine our clinical data. This year we are fielding requests, 'when can I get it,' 'I want it as soon as possible in my cath lab,' 'when will I have access to these products in the USA?' Interest was high before Dr. Gaspardone presented his data and fielded questions on his experience using the NobleStitch™ EL, and after he spoke we were flooded with interest."
Dr. Achille Gaspardone spoke during a moderated session on PFO Closure using the NobleStitch™ EL. Dr. Gaspardone, M.D. (San Eugenio Hospital, Rome, Italy) presented data from several centers in Italy utilizing the NobleStitch™ EL system for PFO closure. Dr. Gaspardone discussed the success of suture-mediated closure using the NobleStitch™ EL stating, "It is quite efficacious, and is possible to carry out in the majority of patients." He continued, "Most importantly, the [system is safe] because there is no metal device left behind particularly on the left side of the heart."
Professor Anthony Nobles, Inventor of the NobleStitch™ EL and Chairman, CEO and Chief Clinical Officer of HeartStitch® which manufactures and distributes the devices, commented: "The demand for our suture-based technology for performing these procedures is growing so rapidly we are trying to open new facilities and hire more staff to keep up with both production of the NobleStitch™ EL, but also to complete the launch of the HeartStitch MR, TA, Pilot and other vascular and cardiovascular products. Physicians continuously expressed to me their desire to have suture-based solutions rather than large metal implants left in the heart. Most physicians that have used the NobleStitch™ EL found it safe, easy to use, and have requested the same suture technologies to replace other implantable clips and devices. With every new physician we proctor the response is universally one of 'I intend to use this in my patients as the first choice for closure. Eliminating the need for an anesthesiologist, we can perform the procedure safely under local anesthesia.' We continue to provide physicians with the tools they are requesting and with the response we have received from the attendees at TCT it is exceeding to see our product's acceptance in the marketplace."
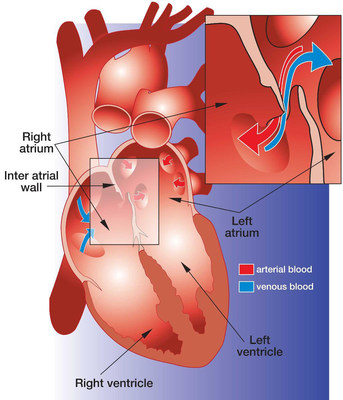
About PFO Closure
A PFO is a relatively common heart defect characterized by an unsealed tunnel between the right and left atria of the heart. This defect has been known to be present in anywhere between 27%-38% of people. However, in a number of cases, it is benign.
The PFO is formed as a trace of the fetal circulation. When the chambers of a human heart begin to develop, a tunnel is made between the right and left atria, allowing blood to flow directly from the venous circulation to the arterial circulation, circumventing the non-functioning fetal lungs. Following birth, the pressure differential between the right and left atria changes with newly operational blood flow to the fully functioning lungs. Because of this, the tunnel eventually closes completely within the first few months.
However, in some patients, the foramen ovale fails to seal and stays "patent." In patients with a Patent Foramen Ovale (PFO), the tunnel can reopen under elevated atrial pressure, such as coughing, or straining.
A key issue with PFO is that it gives a pathway for blood clots to pass directly to the arterial circulation without being filtered out by the capillary bed of the lungs. A PFO can also let deoxygenated blood and certain chemicals cross over to the arterial side. The presence of a PFO has been linked to a number of clinical issues, mainly strokes, migraines and chronic fatigue. Developments are being made to solidify the link between PFO and strokes or migraines, and to identify patients that would benefit from PFO closure.
About HeartStitch®
HeartStitch® Inc. was founded by Prof. Anthony Nobles with the intent of leveraging its technologies in the structural heart marketplace. HeartStitch® is focused on innovative suture-based systems for remotely providing suture repair of structural heart defects and other vascular structures.
The HeartStitch® TA and HeartStitch® MR are FDA cleared for vascular suturing in the United States. HeartStitch® manufactures and markets the NobleStitch™ EL under exclusive license from Nobles Medical technologies II, Inc. NobleStitch™ EL is FDA cleared for vascular suturing in the United States and CE Marked for cardio-vascular suturing and PFO closure in the European Union and the Republic of Kazakhstan, respectively.
HeartStitch® is a registered trademark of HeartStitch, Inc.
HeartStitch® TA for cardiac suturing and transapical access and closure
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8348962, 8469975, 8496676, and 8709020.
HeartStitch® MR for suturing an anatomical valve
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8348962, 8469975, 8496676, 8709020, and 8771296.
For more on HeartStitch® visit http://www.heartstitch.com
For more information, please contact shareholder representatives:
USA
Dru Dobbs
P. +1 714 427 6348
F. +1 714 427 6343
ddobbs@HeartStitch.com
In Kazakhstan
Kazbek Aubakirov
P. +7 777 5009005
kaubakirov@HeartStitch.com
About Nobles Medical Technology II
Nobles Medical Technology II, Inc. was founded by Prof. Anthony Nobles with the intent of leveraging its technologies in the PFO, ASD-closure, and cardiovascular-suturing marketplace. The company does business under the name of Nobles Medical II (NMT II). Initial efforts of the company have been focused in Europe on the innovative suture-based PFO closure system for closing the Patent Foramen Ovale (PFO), a tunnel between the right and left atria of the heart.
The NobleStitch™ is approved for PFO Closure and Cardiovascular suturing in the European Union.
The NobleStitch™ EL is FDA cleared for Vascular and Cardiovascular suturing in the United States. Further information including warnings and precautions can be found in the instructions for use.
NobleStitch™ EL is distributed worldwide by HeartStitch®, Inc. (HeartStitch® is a registered trademark of HeartStitch, Inc.).
NobleStitch™ EL for PFO closure
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8197510, 8246636, 8348962, 8372089, 8469975, 8496676, 8709020, and 9131938.
For more on Nobles Medical Technologies II visit http://www.noblesmed2.com.
For more information, please contact shareholder representatives:
Dru Dobbs
P. +1 714 427 6348
F. +1 714 427 6343
ddobbs@noblesmed2.com


SOURCE Nobles Medical Technology II, Inc.