

PHILADELPHIA, April 26, 2016 /PRNewswire/ -- Results of a long-term follow up study on patients with spinal cord injuries implanted with the NeuRx® Diaphragm Pacing System (DPS) were presented at the 2016 American Spinal Injury Association (ASIA) Scientific Meeting.
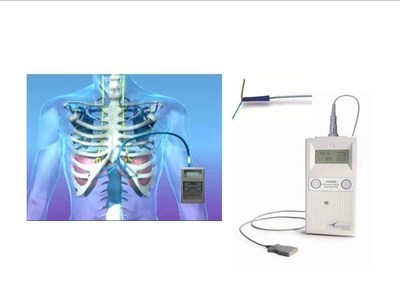
In collaboration with six of the Spinal Cord Injury Model System (SCIMS) centers, Dr. Dan Lammertse of Craig Hospital reported on the level of satisfaction for patients with spinal cord injury who were implanted with the NeuRx® Diaphragm Pacing System (DPS) over the course of seven years. These patients' injuries occurred anywhere from less than one month to up to 28 years prior to implant. The implantation of the device was done, for most patients, with one day of hospitalization with the majority being discharged within two days of implantation. Almost all patients (96%) said they were "happy" to "very happy" with their decision to have the system implanted and a majority experienced an increase in their activity level. Researchers concluded that using the NeuRx DPS® was beneficial, reasonably safe to implant and has strong patient satisfaction support.
"We are very pleased with the striking vote of confidence presented in these results, it confirms what we have been hearing from the users of our device for over a decade", said Anthony R. Ignagni, President & CEO of Synapse Biomedical. "We continue to strive to have a meaningful impact for properly selected spinal cord injured and ALS patients with over 1,500 users of our device since 2002".
Raymond Onders MD, Professor of Surgery and Chair of Surgical Innovation at University Hospitals Case Medical Center, who has been involved with DPS since its inception also presented his latest research that shows that DPS was successful and overwhelmingly preferred by spinal cord injured patients who had only been dependent on part time non-invasive ventilation.
About NeuRx DPS® Technology
NeuRx DPS® (NeuRx DPS®) is a four-channel, battery-powered neurostimulator with implanted electrodes. The device provides electrical stimulation to the muscle and nerves of the diaphragm. The NeuRx DPS® received CE Marking (CE Registration #518356) on November 20, 2007 and is approved for treating patients with diaphragm dysfunction in the European Union. The NeuRx DPS® received FDA approval for ventilator dependency from spinal cord injury on June 17, 2008. In Spinal Cord Injury (SCI), the NeuRx DPS® provides ventilator support in patients with diaphragm dysfunction of neuromuscular origin. Diaphragm dysfunction can result in abnormal or absent respiration in patient populations of high-level spinal cord injury and other injuries or diseases affecting the neuromuscular respiratory pathways. The NeuRx DPS® received FDA approval for treating chronic hypoventilation from ALS on September 28, 2011. The NeuRx DPS® has demonstrated that it can help people with ALS live longer and sleep better than the current standard of care alone.
For more information please visit www.synapsebiomedical.com/products/patientInfo.shtml
Photo - http://photos.prnewswire.com/prnh/20160426/360251
SOURCE Synapse Biomedical Inc.