

Company to showcase poignant and impactful photographs at the Association for Vascular Access 2023 Conference, bringing awareness to IV injuries that are often minimized and ignored
PORTLAND, Ore. , Oct. 13, 2023 /PRNewswire/ -- ivWatch, LLC, the IV safety company, today announced its launch of a gallery exhibit entitled "Pain Should Not Be Silenced" in its booth at the Association for Vascular Access annual conference being held here from October 14-17th. The campaign seeks to move beyond the artifice of medical advertising and pay tribute to those who have suffered from severe IV extravasation injuries. These injuries are usually hidden from the public. Today, clinicians, patients, and parents are courageous – they want to educate, empower, and have a voice to change these outcomes instead of turning a blind eye.
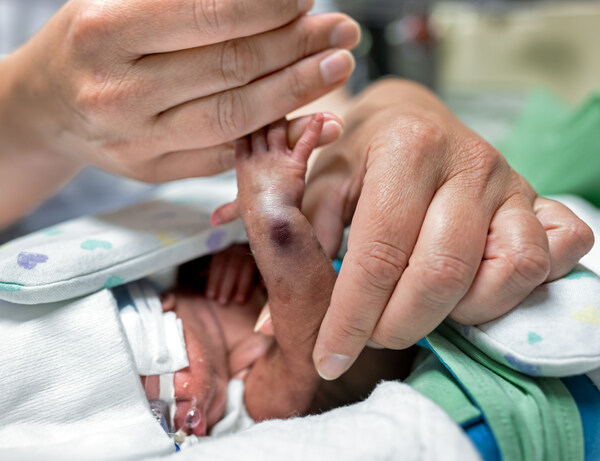
The gallery exhibit is designed to show the human realities and devastations of IV extravasation injuries, specifically in NICU settings. At a time when nursing staffing shortages are rife both in the U.S. and around the world, ivWatch commissioned these images to demonstrate the pain and suffering that can be caused when insufficient monitoring of IV efficacy occurs.
Created in partnership with a famous sports photographer who endured an IV extravasation as a NICU baby, these images pay homage to the incredible amount of suffering these tiny humans will fight their entire lives – not just physically, but mentally as well - as a result of these injuries incurred at such a young age. Each neonate has a story to be told and their parents want to bring awareness to this global crisis so no baby will experience these types of preventable injuries ever again.
"Each of these tiny babies and their parents have a story to tell about these preventable injuries that cause so much pain and suffering. With these images, we set out to pay tribute, honor, and elevate the global IV extravasation crisis to where it belongs – above clinical standards, above job titles, above convention," said Erin Wendell, Chief Marketing Officer at ivWatch. "Our unique approach was to create an unexpected gallery of the harsh realities created by IV injuries, which happen every single day. Our gallery is a reminder that these patients, their parents, and their clinicians need a voice raised to end the culture of silence in which these injuries are often minimized, ignored, or even misdiagnosed."
The suite of images in the gallery represents a view through the eyes of the clinician, a first-hand account of what is seen in the patient population globally and daily. This is just one of the images that will be showcased in the exhibition.
The widely used Helm 2015 clinical paper "Accepted but Unacceptable: peripheral IV catheter failure," still stands true highlighting infiltration rates median at 23.9% and newer publications have published rates in the NICU setting as high as 67% - 78%.1, 2, 3
In addition to the exhibition, the company will be showcasing its IV patient monitoring technology. Company executives and key customers will be presenting at the conference as follows:
ivWatch technology is the only FDA cleared and CE marked device that can detect IV extravasations. A predictive proprietary algorithm and multi-spectral light are designed to provide continuous IV site monitoring to detect fluid leaking outside of the vessel at its earliest stages. Periodic clinical observations combined with smart 24/7 site surveillance technology is proven to reduce infiltration rates in a variety of clinical settings and improve patient outcomes. To learn more about the ivWatch patient monitoring system for IV infiltrations and extravasations visit: https://www.ivwatch.com/tech-overview/.
1Helm, R. E., Klausner, J. D., Klemperer, J. D., Flint, L. M., & Huang, E. (2015). Accepted but unacceptable: peripheral IV catheter failure.
Journal of infusion nursing: the official publication of the Infusion Nurses Society, 38(3), 189–203. https://doi.org/10.1097/NAN.0000000000000100
2Legematt M., et al. Peripheral intravenous cannulation: complication rates in the neonatal population: a multicenter observational study. J Vasc Access. 2016 Jul; 17(4).
3Pettit J., et al. Assessment of the infant with a peripheral intravenous device. Adv Neonatal Care. 2003 Oct;3(5).
About ivWatch, LLC
ivWatch, LLC is a biosensor technology company focused on improving patient safety and the effectiveness of intravenous therapy. Our dedicated and passionate team is pioneering the use of optical sensors to detect adverse IV events early to minimize the risk of injury caused by infiltrations and extravasations. By using this technology, clinicians can leverage continuous monitoring to help identify infiltrations as early as possible. Our innovative IV monitoring solutions are backed by decades of clinical research and device development. To learn more, follow us on Twitter @ivWatch, Facebook @ivWatchLLC, Instagram @ivWatchLLC and LinkedIn @ivWatch-LLC, or visit www.ivWatch.com.
SOURCE ivWatch, LLC