

CHICAGO, Oct. 30, 2019 /PRNewswire/ -- A landmark study presented at the American College of Gastroenterology (ACG) 2019 Annual Scientific Meeting is shedding new light on how compounds that are structurally identical to human milk oligosaccharides (HMOs) can significantly improve IBS symptoms within four weeks.
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8622851-glycom-study-shows-hmo-supplements-like-holigos-improve-ibs-symptoms/
IBS is a chronic disorder that affects the large intestine and can cause cramping, abdominal pain, bloating, gas, diarrhea and/or constipation. ACG reports that while an estimated 10-15% of adults in the U.S. suffer from IBS, only about half have been diagnosed. In recent years, research has linked IBS with imbalances in gut microbiota, a "community" of microorganisms, or bacteria, found in the gastrointestinal (GI) tract.
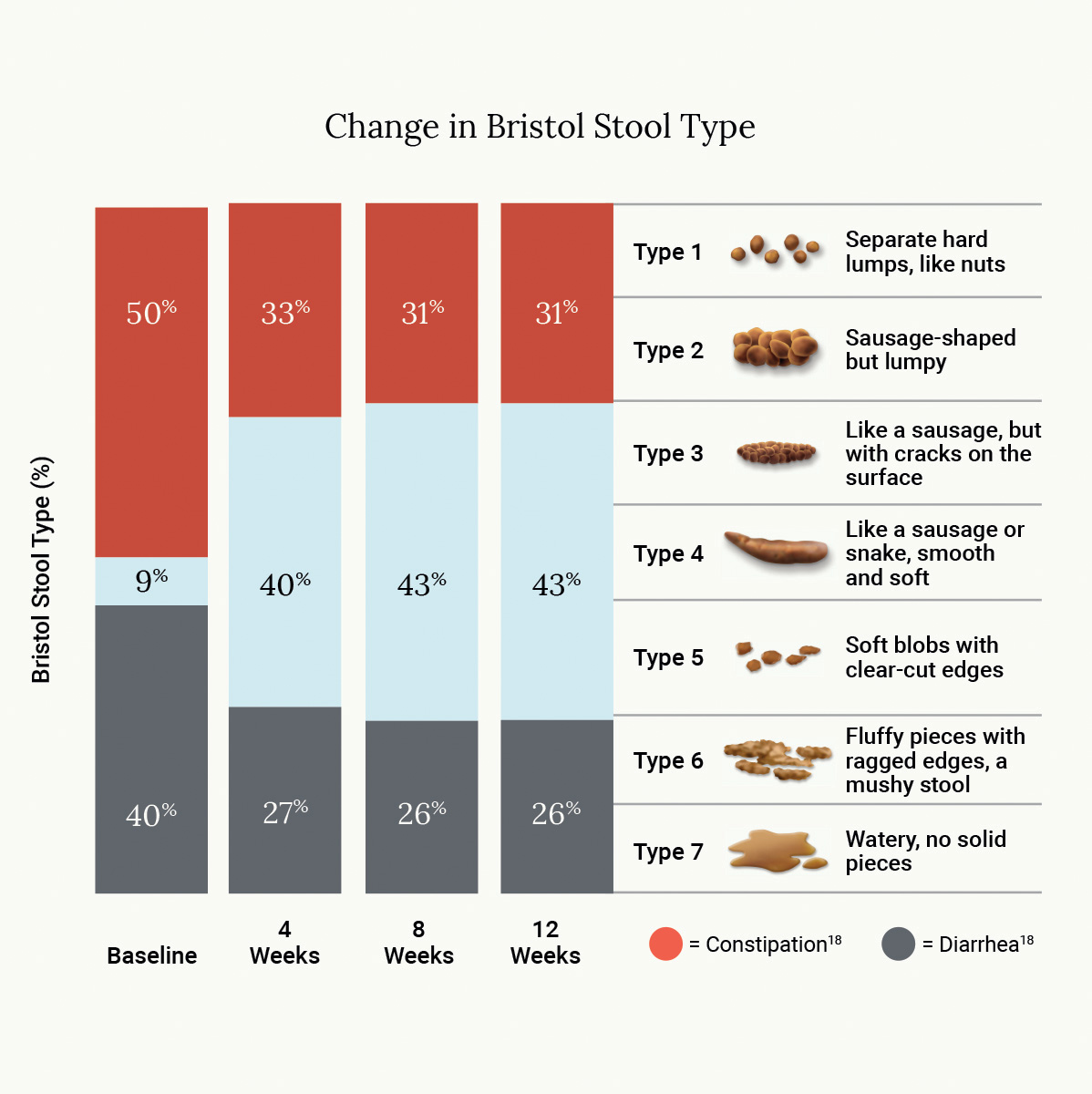
Across 317 patients who enrolled in the 12-week trial, 77% reported significant improvement in symptom severity within four weeks. At 12 weeks, 87% saw a clinically significant reduction in symptom severity. Specific symptom reductions include:
Additionally, the symptom reductions led to a 48% increase in quality of life.
Research has shown that the bacterial composition of the gut may differ in people with IBS. HMOs selectively nourish the "good bacteria," enabling them to flourish and improve the body's gut barrier. The findings in this new study support the growing acceptance in the medical community of microbiota-directed therapies, such as the inclusion of prebiotic HMOs in IBS dietary planning.
The study, "Human Milk Oligosaccharides Improve All the Central Symptoms of Irritable Bowel Syndrome: A Multi-Center, Open Label Trial," was presented at ACG 2019 during a plenary session by Magnus Simrén, MD, PhD, professor and senior consultant at the department of internal medicine and clinical nutrition for the Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Sweden. Dr. Simrén is also an adjunct professor of medicine at the University of North Carolina School of Medicine in Chapel Hill.
ACG is the pre-eminent professional organization that champions the prevention, diagnosis and treatment of digestive disorders through compassionate, evidence-based care.
Patients in the study were given a unique formulation of two types of HMOs found in Holigos® (hōly-gōs) IBS, a medical food produced by Glycom. Medical foods are intended for the specific dietary management of a disease or condition that has distinctive nutritional requirements which cannot be met by normal diet alone.
While Holigos® IBS is available without a prescription, it should be taken under medical supervision. The active ingredients have been positively reviewed by the FDA and accepted as safe. For more information about the proprietary blend of HMOs used in the study, visit holigos.com.
About Glycom
Glycom is a global biotechnology company dedicated to the scientific, clinical and commercial development of human milk oligosaccharides (HMOs) for a broad range of health applications. Glycom, headquartered in Hørsholm, Denmark, is the world's leading supplier of HMOs and has a U.S. facility located in Covington, La. Glycom is privately held. For more information, visit glycom.com.
CONTACT INFO:
Marita Gomez 630-936-9105



SOURCE Glycom
