

BOULDER, Colo., Jan. 14, 2019 /PRNewswire/ -- Array BioPharma Inc. (Nasdaq: ARRY) today announced updated safety and efficacy results, including mature overall survival (OS), from the safety lead-in of the Phase 3 BEACON CRC trial evaluating the triplet combination of BRAFTOVI® (encorafenib), a BRAF inhibitor, MEKTOVI® (binimetinib), a MEK inhibitor and ERBITUX® (cetuximab), an anti-EGFR antibody, in patients with BRAFV600E-mutant metastatic colorectal cancer (mCRC). The results showed that mature median OS was 15.3 months (95% CI, 9.6–not reached) for patients treated with the triplet. These data will be presented on Saturday, January 19 at the ASCO 2019 Gastrointestinal Cancers Symposium in San Francisco, California.
Updated median progression-free survival (mPFS) and updated confirmed overall response rate (ORR) results for patients treated with the triplet in the safety lead-in remain the same, as previously reported, with 8 months mPFS (95% CI, 5.6-9.3) and a 48% ORR (95% CI, 29.4–67.5). Among the 17 patients who received only one prior line of therapy, the ORR was 62%.
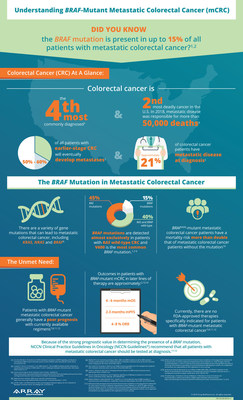
A BRAF mutation is present in up to 15% of all patients with mCRC and V600 is the most common BRAF mutation. [1-5] BRAFV600E-mutant mCRC patients have a mortality risk more than double that of mCRC patients without the mutation, and currently there are no U.S. Food and Drug Administration (FDA)-approved therapies specifically indicated for this high unmet need population. [3-10]
"The mature median overall survival of 15.3 months demonstrated in the safety lead-in of the BEACON CRC trial is unprecedented in this patient population and, for context, represents a substantial improvement compared to the observed historical published benchmarks of approximately 4 to 6 months for median overall survival with current standards of care in patients with BRAF-mutant mCRC," said Axel Grothey, M.D., BEACON CRC trial lead investigator and Co-Chair of the National Cancer Institute's Gastrointestinal Cancer Steering Committee, West Cancer Center, Memphis, TN. "These updated data further underscore the potential of this triplet for patients with BRAF-mutant mCRC who are in desperate need of effective new treatment options."
The triplet combination was generally well-tolerated with no unexpected toxicities. The most common grade 3 or 4 adverse events seen in at least 10% of patients were fatigue (13%), anemia (10%), increased creatine phosphokinase (10%), increased aspartate aminotransferase (10%) and urinary tract infections (10%). The rate of grade 3 or 4 skin toxicities continued to be lower than generally observed with ERBITUX in mCRC.
"We are delighted with the updated results from the BEACON CRC safety lead-in. Following consultations with the FDA and European Medicines Agency, we initiated an amendment to the BEACON CRC protocol to allow for an interim analysis based primarily on confirmed ORR and durability of response endpoints, which we believe could support an accelerated approval with positive results," said Victor Sandor, M.D., Chief Medical Officer, Array BioPharma. "We anticipate topline results from this interim analysis in the first half of this year. This timing allows for the subset of patients required for the interim analysis of ORR to achieve a response and for the durability of responses to be appropriately evaluated."
On August 7, 2018, Array announced that the FDA granted Breakthrough Therapy Designation to BRAFTOVI, in combination with MEKTOVI and ERBITUX for the treatment of patients with BRAFV600E-mutant mCRC as detected by an FDA-approved test, after failure of one to two prior lines of therapy for metastatic disease.
The triplet combination of BRAFTOVI, MEKTOVI and ERBITUX for the treatment of patients with BRAFV600E-mutant mCRC is investigational and not approved by the FDA.
BEACON CRC Safety Lead-In Data | |
Title: | Abstract #688: Updated results of the BEACON CRC safety lead-in: Encorafenib (ENCO) + binimetinib (BINI) + cetuximab (CETUX) for BRAFV600E-mutant metastatic colorectal cancer (mCRC) |
Presenter: | Scott Kopetz, M.D., Department of Gastrointestinal Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center |
Date: | Saturday, January 19, 2019 |
Times: | 7:00 - 7:55 a.m. and 12:15 - 1:45 p.m. Pacific Time |
Location: | Poster N13; Moscone West Building |
Following the presentation, the slides will be available as a PDF on the Publications section of the Array website.
Array will host an investor webcast presentation of the BEACON CRC safety lead-in data.
Investor Webcast: | |
Presenter: | Axel Grothey, M.D., BEACON CRC trial lead investigator and Co-Chair of the National Cancer Institute's Gastrointestinal Cancer Steering Committee, West Cancer Center, Memphis, TN |
Date: | Tuesday, January 15 |
Time: | 9:00 a.m. Eastern Time |
Toll-Free: | (844) 464-3927 |
Toll: | (765) 507-2598 |
Pass Code: | 6774596 |
Webcast, including replay and conference call slides: https://edge.media-server.com/m6/p/y85tt94b
About Colorectal Cancer
Worldwide, colorectal cancer is the third most common type of cancer in men and the second most common in women, with approximately 1.4 million new diagnoses in 2012. Globally in 2012, approximately 694,000 deaths were attributed to colorectal cancer. [11] In the U.S. alone, an estimated 140,250 patients will be diagnosed with cancer of the colon or rectum in 2018, and approximately 50,000 are estimated to die of their disease. [12] BRAF mutations are estimated to occur in up to 15% of patients with mCRC and represent a poor prognosis for these patients. [1-5] The V600 mutation is the most common BRAF mutation and the risk of mortality in CRC patients with the BRAFV600E mutation is more than two times higher than for those with wild-type BRAF. [1,10,13] Several irinotecan and cetuximab-containing regimens, similar to the BEACON CRC control arm, have established observed historical published benchmarks in BRAFV600E-mutant mCRC patients, whose disease has progressed after one or two prior lines of therapy. These benchmarks include ORR of 4% to 8%, mPFS of 2 to 3 months and median OS of 4 to 6 months. [3-9,14] BRAF V600E-mutant mCRC is an area of high unmet need as there are currently no FDA-approved therapies specifically indicated for patients with BRAF-mutant mCRC, and these patients derive limited benefit from available chemotherapy regimens. [15-17] For more information about BRAFV600E-mutant mCRC visit www.brafmcrc.com.
About BEACON CRC
BEACON CRC is a randomized, open-label, global trial evaluating the efficacy and safety of BRAFTOVI, MEKTOVI and ERBITUX in patients with BRAFV600E-mutant mCRC whose disease has progressed after one or two prior regimens. BEACON CRC is the first and only Phase 3 trial designed to test a BRAF/MEK combo targeted therapy in BRAFV600E-mutant mCRC. Thirty patients were treated in the safety lead-in and received the triplet combination (BRAFTOVI 300 mg daily, MEKTOVI 45 mg twice daily and ERBITUX per label). Of the 30 patients, 29 had a BRAFV600 mutation. MSI-H, resulting from defective DNA mismatch repair, was detected in only 1 patient. As previously announced, the triplet combination demonstrated good tolerability, supporting initiation of the randomized portion of the trial. The randomized portion of the BEACON CRC trial is designed to assess the efficacy of BRAFTOVI in combination with ERBITUX with or without MEKTOVI compared to ERBITUX and irinotecan-based therapy. Approximately 615 patients are expected to be randomized 1:1:1 to receive triplet combination, doublet combination (BRAFTOVI and ERBITUX) or the control arm (irinotecan-based therapy and ERBITUX). The study has been amended to include an interim analysis of endpoints including ORR. The primary overall survival endpoint is a comparison of the triplet combination to the control arm. Secondary endpoints address efficacy of the doublet combination compared to the control arm, and the triplet combination compared to the doublet therapy. Other secondary endpoints include PFS, duration of response, safety and tolerability. Health related quality of life data will also be assessed. The trial is being conducted at over 200 investigational sites in North America, South America, Europe and the Asia Pacific region. The BEACON CRC trial is being conducted with support from Ono Pharmaceutical Co., Pierre Fabre and Merck KGaA, Darmstadt, Germany (support is for sites outside of North America).
About BRAFTOVI + MEKTOVI
BRAFTOVI is an oral small molecule BRAF kinase inhibitor and MEKTOVI is an oral small molecule MEK inhibitor which target key enzymes in the MAPK signaling pathway (RAS-RAF-MEK-ERK). Inappropriate activation of proteins in this pathway has been shown to occur in many cancers including melanoma, colorectal cancer, non-small cell lung cancer and others. In the U.S., BRAFTOVI + MEKTOVI are approved for the treatment of unresectable or metastatic melanoma with a BRAFV600E or BRAFV600K mutation, as detected by an FDA-approved test. BRAFTOVI is not indicated for treatment of patients with wild-type BRAF melanoma. In Europe, the combination is approved for adult patients with unresectable or metastatic melanoma with a BRAFV600 mutation, as detected by a validated test.
Array has exclusive rights to BRAFTOVI and MEKTOVI in the U.S. and Canada. Array has granted Ono Pharmaceutical exclusive rights to commercialize both products in Japan and South Korea, Medison exclusive rights to commercialize both products in Israel and Pierre Fabre exclusive rights to commercialize both products in all other countries, including Europe, Latin American and Asia (excluding Japan and South Korea).
BRAFTOVI + MEKTOVI have received regulatory approval in the United States, European Union, and Japan. The Swiss Medicines Agency (Swissmedic) is currently reviewing the Marketing Authorization Applications for BRAFTOVI and MEKTOVI submitted by Pierre Fabre.
Indications and Usage
BRAFTOVI® (encorafenib) and MEKTOVI® (binimetinib) are kinase inhibitors indicated for use in combination for the treatment of patients with unresectable or metastatic melanoma with a BRAFV600E or BRAFV600K mutation, as detected by an FDA-approved test.
Limitations of Use: BRAFTOVI is not indicated for the treatment of patients with wild-type BRAF melanoma.
BRAFTOVI + MEKTOVI Important Safety Information
The information below applies to the safety of the combination of BRAFTOVI and MEKTOVI unless otherwise noted. See full Prescribing Information for BRAFTOVI and for MEKTOVI for dose modifications for adverse reactions.
Warnings and Precautions
New Primary Malignancies: Cutaneous and non-cutaneous malignancies can occur. In the COLUMBUS trial, cutaneous squamous cell carcinoma, including keratoacanthoma, occurred in 2.6% and basal cell carcinoma occurred in 1.6% of patients. Perform dermatologic evaluations prior to initiating treatment, every 2 months during treatment, and for up to 6 months following discontinuation of treatment. Manage suspicious skin lesions with excision and dermatopathologic evaluation. Dose modification is not recommended for new primary cutaneous malignancies. Based on its mechanism of action, BRAFTOVI may promote malignancies associated with activation of RAS through mutation or other mechanisms. Monitor patients receiving BRAFTOVI for signs and symptoms of non-cutaneous malignancies. Discontinue BRAFTOVI for RAS mutation-positive non-cutaneous malignancies.
Tumor Promotion in BRAF Wild-Type Tumors: Confirm evidence of BRAFV600E or V600K mutation prior to initiating BRAFTOVI.
Cardiomyopathy, manifesting as left ventricular dysfunction associated with symptomatic or asymptomatic decreases in ejection fraction, has been reported in patients. In the COLUMBUS trial, cardiomyopathy occurred in 7% and Grade 3 left ventricular dysfunction occurred in 1.6% of patients. Cardiomyopathy resolved in 87% of patients. Assess left ventricular ejection fraction by echocardiogram or MUGA scan prior to initiating treatment, 1 month after initiating treatment, and then every 2 to 3 months during treatment. Safety has not been established in patients with a baseline ejection fraction that is either below 50% or below the institutional lower limit of normal. Patients with cardiovascular risk factors should be monitored closely.
Venous Thromboembolism (VTE): In the COLUMBUS trial, VTE occurred in 6% of patients, including 3.1% of patients who developed pulmonary embolism.
Hemorrhage: In the COLUMBUS trial, hemorrhage occurred in 19% of patients and ≥ Grade 3 hemorrhage occurred in 3.2% of patients. Fatal intracranial hemorrhage in the setting of new or progressive brain metastases occurred in 1.6% of patients. The most frequent hemorrhagic events were gastrointestinal, including rectal hemorrhage (4.2%), hematochezia (3.1%), and hemorrhoidal hemorrhage (1%).
Ocular Toxicities: In the COLUMBUS trial, serous retinopathy occurred in 20% of patients; 8% were retinal detachment and 6% were macular edema. Symptomatic serous retinopathy occurred in 8% of patients with no cases of blindness. RVO is a known class-related adverse reaction of MEK inhibitors and may occur in patients treated with MEKTOVI in combination with encorafenib. In patients with BRAF mutation-positive melanoma across multiple clinical trials, 0.1% of patients experienced retinal vein occlusion (RVO). The safety of MEKTOVI has not been established in patients with a history of RVO or current risk factors for RVO including uncontrolled glaucoma or a history of hyperviscosity or hypercoagulability syndromes. Perform ophthalmological evaluation for patient-reported acute vision loss or other visual disturbance within 24 hours. Permanently discontinue MEKTOVI in patients with documented RVO. In COLUMBUS, uveitis, including iritis and iridocyclitis was reported in 4% of patients. Assess for visual symptoms at each visit. Perform ophthalmological evaluation at regular intervals and for any visual disturbances, and to follow new or persistent ophthalmologic findings.
Interstitial Lung Disease (ILD): ILD, including pneumonitis occurred in 0.3% of patients with BRAF mutation-positive melanoma across multiple clinical trials. Assess new or progressive unexplained pulmonary symptoms or findings for possible ILD.
Hepatotoxicity: In the COLUMBUS trial, the incidence of Grade 3 or 4 increases in liver function laboratory tests was 6% for alanine aminotransferase (ALT) and 2.6% for aspartate aminotransferase (AST), and 0.5% for alkaline phosphatase. Monitor liver laboratory tests before and during treatment and as clinically indicated.
Rhabdomyolysis: In the COLUMBUS trial, elevation of laboratory values of serum creatine phosphokinase (CPK) occurred in 58% of patients. Rhabdomyolysis was reported in 0.1% of patients with BRAF mutation-positive melanoma across multiple clinical trials. Monitor CPK and creatinine levels prior to initiating MEKTOVI, periodically during treatment, and as clinically indicated.
QTc Prolongation: BRAFTOVI is associated with dose-dependent QTc interval prolongation in some patients. In the COLUMBUS trial, an increase in QTcF to > 500 ms was measured in 0.5% (1/192) of patients. Monitor patients who already have or who are at significant risk of developing QTc prolongation. Correct hypokalemia and hypomagnesemia prior to and during BRAFTOVI administration. Withhold, reduce dose, or permanently discontinue for QTc > 500 ms.
Embryo-Fetal Toxicity: BRAFTOVI or MEKTOVI can cause fetal harm when administered to pregnant women. BRAFTOVI can render hormonal contraceptives ineffective. Non-hormonal contraceptives should be used during treatment and for at least 30 days after the final dose for patients taking BRAFTOVI + MEKTOVI.
Adverse Reactions
The most common adverse reactions (≥20%, all Grades, in the COLUMBUS trial): were fatigue, nausea, diarrhea, vomiting, abdominal pain, arthralgia, myopathy, hyperkeratosis, rash, headache, constipation, visual impairment, serous retinopathy.
In the COLUMBUS trial, the most common laboratory abnormalities (≥20%, all Grades): included increased creatinine, increased CPK, increased gamma glutamyl transferase, anemia, increased ALT, hyperglycemia, increased AST, and increased alkaline phosphatase.
Drug Interactions
Avoid concomitant use of strong or moderate CYP3A4 inhibitors or inducers and sensitive CYP3A4 substrates with BRAFTOVI. Modify BRAFTOVI dose if concomitant use of strong or moderate CYP3A4 inhibitors cannot be avoided. Avoid co-administration of BRAFTOVI with medicinal products with a known potential to prolong QT/QTc interval.
Please see full Prescribing Information for BRAFTOVI and full Prescribing Information for MEKTOVI for additional information. [18,19] You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch. You may also report side effects to Array at 1-844-Rx-Array (1-844-792-7729).
About Array BioPharma
Array BioPharma Inc. is a fully-integrated, biopharmaceutical company focused on the discovery, development and commercialization of transformative and well-tolerated targeted small molecule drugs to treat patients afflicted with cancer and other high-burden diseases. Array markets BRAFTOVI® (encorafenib) capsules in combination with MEKTOVI® (binimetinib) tablets for the treatment of patients with unresectable or metastatic melanoma with a BRAFV600E or BRAFV600K mutation in the United States and with partners in other major worldwide markets. Array's lead clinical programs, encorafenib and binimetinib, are being investigated in over 30 clinical trials across a number of solid tumor indications, including a Phase 3 trial in BRAF-mutant metastatic colorectal cancer. Array's pipeline includes several additional programs being advanced by Array or current license-holders, including the following programs currently in registration trials: selumetinib (partnered with AstraZeneca), LOXO-292 (partnered with Loxo Oncology), ipatasertib (partnered with Genentech), tucatinib (partnered with Seattle Genetics) and ARRY-797. Vitrakvi® (larotrectinib, partnered with Loxo Oncology) is approved in the United States and Ganovo® (danoprevir, partnered with Roche) is approved in China. For more information on Array, please visit www.arraybiopharma.com or follow @arraybiopharma on Twitter and LinkedIn.
References
[1] Sorbye, et al., PLoS One. 2015.
[2] Vecchione, et al., Cell. 2016.
[3] Saridaki, et al., PLoS One. 2013.
[4] Loupakis, et al., Br J Cancer. 2009.
[5] Corcoran, et al., Cancer Discovery. 2012
[6] Kopetz, et al., ASCO 2017.
[7] De Roock, et al., Lancet Oncol. 2010.
[8] Ulivi, et al., J Transl Med. 2012.
[9] Peeters, et al., ASCO 2014.
[10] Ardekani, et al., PLoS One. 2012.
[11] Global Cancer Facts & Figures 3rd Edition. American Cancer Society. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-3rd-edition.pdf. Accessed January 2018.
[12] Cancer Facts & Figures 2018. American Cancer Society. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed January 2018.
[13] Safaee, et al., PLoS One. 2012.
[14] Seymour, et al., Lancet Oncol. 2013 (supplementary appendix).
[15] NCCN Clinical Practice Guidelines in Oncology for Colon Cancer. Version 4.2018. National Comprehensive Cancer Network.
[16] Van Cutsem, et al., Annals of Oncology. 2016.
[17] Ursem, et al., Gastrointest Cancer, 2018.
[18] BRAFTOVI® (encorafenib) Prescribing Information. Array BioPharma Inc., June 2018.
[19] MEKTOVI® (binimetinib) Prescribing Information. Array BioPharma Inc., June 2018.
Forward-Looking Statement
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, among others, statements about the future development plans of encorafenib and binimetinib; expectations that events will occur that will create greater value for Array; and the potential for the results of current and future clinical trials to support regulatory approval or the marketing success of encorafenib and binimetinib. Because these statements reflect our current expectations concerning future events and involve significant risks and uncertainties, our actual results could differ materially from those anticipated in these forward-looking statements as a result of many factors. These factors include, but are not limited to, the potential that the FDA, EMA or other regulatory agencies determine results from clinical trials are not sufficient to support registration or marketing approval of encorafenib and binimetinib; our ability to effectively and timely conduct clinical trials in light of increasing costs and difficulties in locating appropriate trial sites and in enrolling patients who meet the criteria for certain clinical trials; risks associated with our dependence on third-party service providers to successfully conduct clinical trials and to manufacture drug substance and product within and outside the U.S.; our ability to grow and successfully develop commercialization capabilities; our ability to achieve and maintain profitability and maintain sufficient cash resources; and our ability to attract and retain experienced scientists and management. Additional information concerning these and other risk factors can be found in our most recent annual report filed on Form 10-K, in our quarterly reports filed on Form 10-Q, and in other reports filed by Array with the Securities and Exchange Commission. We are providing this information as of January 14, 2019. We undertake no duty to update any forward-looking statements to reflect the occurrence of events or circumstances after the date of such statements or of anticipated or unanticipated events that alter any assumptions underlying such statements.
BRAFTOVI® and MEKTOVI® are registered trademarks of Array BioPharma Inc. in the United States and various other countries. Vitrakvi® is a registered trademark of Bayer AG. All trademarks are properties of their respective owners.
Statements attributed to Dr. Grothey are his alone and do not reflect the opinion of the National Cancer Institute.
CONTACTS:
Investor Relations
Array BioPharma
Andrea N. Flynn, Ph.D.
Senior Director, Investor Relations & Corporate Communications
(303) 381-6600
ir@arraybiopharma.com
Media
Y&R PR
Erika Hackmann, Media Relations
212-303-2305
erika.hackmann@yr.com

SOURCE Array BioPharma Inc.
