

EVERETT, Wash., June 8, 2017 /PRNewswire/ -- Fluke Biomedical will present in-depth product showcases on defibrillator/AED testing and electrical safety at the Association for the Advancement of Medical Instrumentation (AAMI) Annual Conference being held June 9-12, at the Convention Center in Austin, Texas.
The product showcases, which will be held in the Expo Hall, are:
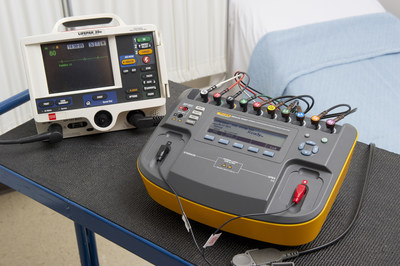
June 10, 1:00pm – Defibrillator Analyzer Testing — Fluke Biomedical Product Manager Ashton Solecki and Global Training Manager Jerry Zion will cover basic defibrillator and AED performance testing. Using the Impulse 7000DP defibrillator analyzer and 7010 Load Box, the session will cover how to conduct accurate performance testing, including impedance tests, and a discussion on AED preventive maintenance.
June 11, 1:00pm – Automating Electrical Safety Testing — Fluke Biomedical Senior Marketing Manager, Shirin Khanna, discusses simplifying electrical safety testing by automating tests with Fluke Biomedical Electrical Safety Analyzers. Shirin will demonstrate how to ensure automatic testing for all required tests are performed the same way each time, leading to improvements in compliance, time savings, and error reduction associated with testing and documentation.
AAMI is the primary source of consensus standards, both national and international, for the medical device industry, as well as practical information, support, and guidance for healthcare technology and sterilization professionals.
For more information on Fluke Biomedical products, visit: www.flukebiomedical.com.
About Fluke Biomedical
Fluke Biomedical is the premier, global provider of test and measurement equipment and services to the healthcare industry. It serves biomedical engineers, quality-assurance technicians, medical physicists, oncologists and radiation-safety professionals and is continually expanding its range of solutions to a broader range of health and safety professionals. For more information, visit www.flukebiomedical.com.
For more information:
Shelly Carothers
shelly.carothers@fluke.com

SOURCE Fluke Biomedical